T4K3.news
Breakthrough CRISPR diabetes therapy shows early success
A 42 year old Swedish patient in a CRISPR edited islet cell trial began producing insulin after 12 weeks, reducing the need for immunosuppressants.

A CRISPR edited transplant offers the first clear hint that type 1 diabetes could be controlled without lifelong immunosuppressants.
Gene edited islet cell transplant cures type 1 diabetes in trial
Scientists report the first human trial of a CRISPR edited islet cell transplant aimed at treating type 1 diabetes. The patient, a 42‑year‑old man from Sweden diagnosed at age five, received islet cells from a living donor that were engineered to match his immune system and avoid rejection. Over 12 weeks, his liver began producing insulin, reducing his dependence on external insulin.
Unlike earlier islet transplants, this approach did not require lifelong immunosuppressants. The trial uses gene edited cells to reduce rejection risk and could lower long term costs associated with immunosuppression. The patient experienced side effects such as vein inflammation and numbness, which resolved; The study notes 8.4 million people live with type 1 diabetes worldwide. The research was published in The New England Journal of Medicine. The cost of traditional islet cell transplants is around 100,000 dollars. The donor cells can come from living or deceased donors. More trials are needed to confirm safety and durability before this can be offered widely.
Key Takeaways
"This trial marks a turning point in how we treat autoimmune disease"
Editorial assessment of significance
"A single patient milestone signals a longer journey for millions"
Caution about scale
"Access and cost will decide whether millions benefit"
Equity concern
If this result is replicated, it could reshape the diabetes treatment landscape by reducing daily management burdens and long term health risks tied to insulin therapy. The CRISPR approach raises safety and regulatory questions, including potential long term durability and off target edits. Donor cell supply and ethical considerations around living donors will also be part of the debate as trials expand.
Cost and access will determine whether this breakthrough translates into real world benefit. Even with science on its side, patients may face high up front costs and complex logistics. Policymakers and insurers will need to weigh potential long term savings against short term expenses. The article reads as cautiously optimistic and invites broader trials that include diverse populations.
Highlights
- This could redefine how we think about immune rejection
- A milestone not a final destination
- Costs and access will decide who benefits
- More trials will tell us about long term safety
Budget and access risks linked to breakthrough therapy
The therapy could be expensive and resource intensive, potentially limiting early access. Without scalable production and comprehensive coverage, benefits may be uneven across populations.
The road ahead will test safety, cost, and access as this science moves forward.
Enjoyed this? Let your friends know!
Related News
Gene edited cells enable insulin production without immunosuppressants
Gene edited islet cell transplant cures type 1 diabetes in Sweden

Weill Family Foundation launches cancer research hub

mRNA research funding under fire

Study shows promise for type 1 diabetes treatment
CAR NK therapy shows promise for autoimmune diseases

New hair loss drug shows promise
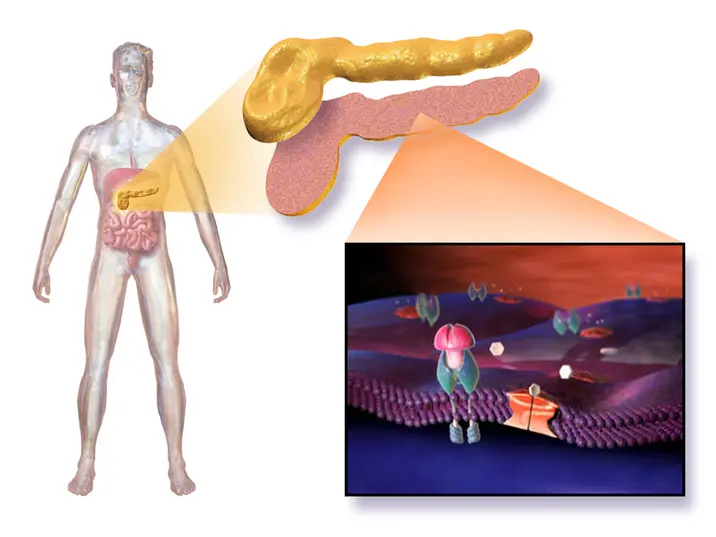
First gene-edited islet transplant succeeds
