T4K3.news
FluMist home delivery expands vaccine access
AstraZeneca launches FluMist home delivery in 34 states after FDA self administration approval
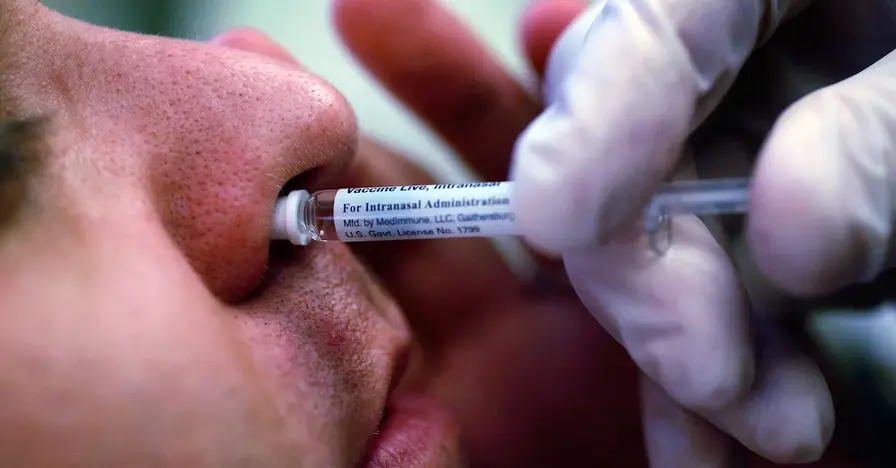
The FluMist nasal spray vaccine can be ordered online for home delivery in 34 states, expanding at home vaccination options.
FluMist Moves Vaccination to Home Delivery
Starting today, FluMist is available through an online pharmacy in 34 states. The FDA approved self administration last year, allowing direct to consumer access while the vaccine remains approved for ages 2 through 49; those under 18 must be administered by a caregiver. Orders require a medical screening questionnaire, a licensed provider review, and insurance verification before a prescription is issued and shipped to the purchaser’s home on a date chosen by the buyer. An $8.99 delivery fee applies, and the vaccine will continue to be available in doctors’ offices and pharmacies where clinicians supervise administration.
The CDC notes the 2024-2025 flu season was high severity across age groups, with at least 610,000 hospitalizations and 27,000 deaths. Vaccination rates lag behind targets, with about 47 percent of adults and 49.2 percent of children receiving a flu shot by late April. AstraZeneca says not all states are covered yet because of local pharmacy laws, but aims to expand to contiguous states in the future and maintain traditional vaccination channels for those who prefer clinics.
Key Takeaways
"Getting vaccinated each year is the best way to prevent influenza, which causes illness in a substantial proportion of the US population every year and may result in serious complications, including hospitalization and death"
Peter Marks, former FDA official, commenting on self administration approval
"We recognized that people's daily lives are busier than ever, and we knew we needed to meet people where they are facing the realities of real life"
Tonya Villafana, AstraZeneca vice president, at a press conference
"FluMist Home is designed to remove traditional obstacles to vaccination"
AstraZeneca statement accompanying the home delivery option
This move reflects a broader shift toward consumer friendly health products that sit at the edge of traditional care. It raises questions about safety oversight and how to ensure proper use when a vaccine is self administered at home. The model mirrors other direct to consumer pharma efforts, which can boost access but also shift responsibility for screening and adherence away from clinicians.
If well regulated, home vaccination could lift vaccination rates by removing common barriers such as scheduling and travel time. The outcome will depend on clear usage instructions, reliable screening, privacy safeguards, and continued public trust in vaccines as a safe and effective public health tool.
Highlights
- Vaccination meets people where they are
- Convenience should not replace safety checks
- A door to door vaccine era has begun
- Access is not the same as acceptance
As vaccines move into home settings, clear guidance and oversight will shape how widely this option is used.
Enjoyed this? Let your friends know!
Related News

AstraZeneca expands FluMist to home delivery

FluMist expands to home vaccination

FluMist Home taps living rooms for flu defense

FluMist Home gains home-use approval
Health policy shifts raise safety and access concerns
RSV vaccines expand access this season

Israeli Forces Face Allegations of War Crimes

Amazon expands perishable delivery
