T4K3.news
FluMist expands to home vaccination
FluMist Home starts rolling out in 34 states this season with an at home option for ages 2 to 49; shipping adds a small cost.

The first self administered flu vaccine is now available to order for home delivery with details on cost age limits and state availability.
FluMist Now Available for At Home Flu Vaccination
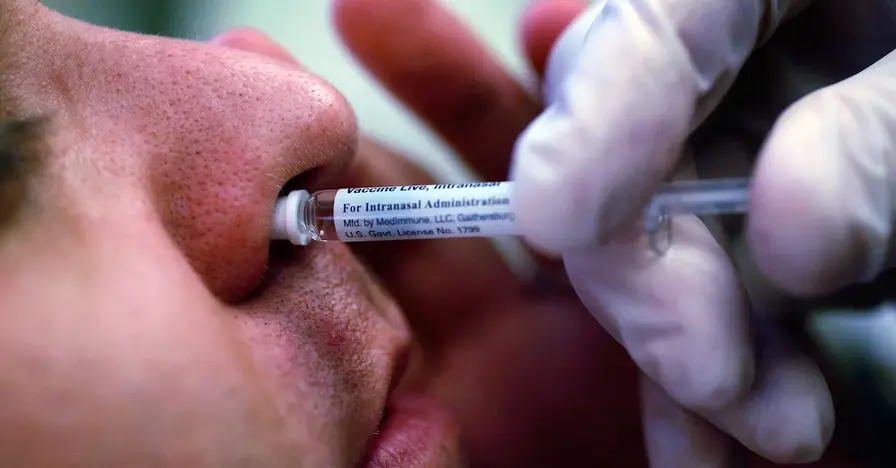
FluMist is the nasal spray version of a flu vaccine and is now offered for self administration at home under the FDA expanded approval. FluMist Home will ship the same nasal spray to customers in 34 states this season, mirroring what patients receive in clinics. The service notes an $8.99 shipping and processing fee, while most commercial insurance will cover the vaccine itself. Ordering requires basic personal and insurance details along with a payment method.
The vaccine is approved for home use by people aged 2 to 49. Caregivers should administer FluMist to children aged 2 through 17. The product cautions against use by individuals with severe allergies to components eggs or to other flu vaccines and directs readers to the full safety information. Availability depends on state law, with 34 states listed and plans to expand to most of the remaining contiguous states in future seasons.
Key Takeaways
"FluMist is approved for at home use for ages 2 to 49"
official safety information on the product page
"Vaccination moves from clinic to living room"
editorial observation on the delivery shift
"Shipping fees temper the promise of home vaccines"
consumer impact note
"Caregivers will play a bigger role in child vaccines"
impact on families and routines
Experts say home administration meets a demand for convenience and could boost vaccination rates among busy families. But it also shifts responsibility to patients and caregivers, increases the risk of incorrect administration, and relies on shipping reliability for timely protection. Privacy and data sharing during online ordering add another layer of consideration.
State by state rules will shape access, and the move raises questions for insurers, clinics, and public health teams. If the model proves safe and scalable, it may become part of a broader trend toward at home care; if not, it risks widening gaps for those without good internet access or stable delivery options. The coming seasons will show how far the idea can go.
Highlights
- Vaccinate at home a new chapter in public health
- Shipping fees temper the promise of home vaccines
- Caregivers take on a bigger role in protection
- Access moves closer to households
At home vaccine raises access and privacy questions
The new home vaccination model could improve convenience but raises privacy and data handling concerns. It depends on state laws, which vary and could limit rollout, creating uneven access. The approach also relies on shipping and online ordering, which may pose logistical risks.
The home vaccine model could redefine how we approach flu protection
Enjoyed this? Let your friends know!
Related News
FluMist home delivery expands vaccine access

AstraZeneca expands FluMist to home delivery

FluMist Home taps living rooms for flu defense

FluMist Home gains home-use approval
RSV vaccines expand access this season

Owain James case prompts call for tissue handling reform

First U.S. death from West Nile Virus reported in Arizona

Myanmar election plan faces global scrutiny