T4K3.news
Covid vaccine update timeline
New vaccines may not be ready until mid September, affecting fall protection plans.
A summer rise in infections comes with uncertainty over when updated vaccines will be available for the fall season.
Covid wave grows as vaccines delayed to mid September
Coronavirus infections are climbing again this summer, marking a new wave as schools reopen and people resume travel. Wastewater data show rising viral levels across the West, South and Midwest, with the trend moving toward the Northeast. Emergency department data indicate infections among children are rising, though overall hospital strain remains lower than in past waves because many people have some level of immunity from prior infections or vaccines.
FDA officials have not yet approved an updated vaccine for fall. A decision is anticipated no earlier than mid September, which means many Americans may rely on the current formula at the start of the season. The plan appears to favor high risk groups first, such as adults 65 and older and people with chronic conditions, before broad access is restored. After FDA approval, the CDC's ACIP will decide who should receive the shots, while insurers commonly cover ACIP recommendations. The current vaccines remain available, though supply could be limited as manufacturers shift toward the new formulation. Experts stress that vaccines remain the best defense against severe disease and death, even as non pharmaceutical measures like masking and ventilation continue to matter in crowded indoor settings. The current dominant variant is XFG, an offshoot of the JN.1 lineage, and regulators have urged updates targeting LP.8.1 to stay ahead of the virus.
Key Takeaways
"The current rise looks similar to seasonal bumps in previous years and is not driving a surge in severe illness."
Kuritzkes on wave pattern
"It's like wearing a seat belt. It doesn't prevent an accident, but it decreases the likelihood of dying from one."
Milstone on vaccines
"Most insurers must pay for ACIP-recommended vaccines."
Insurance coverage status
"The FDA may not sign off on Pfizer’s updated coronavirus vaccine for children six months to four years old."
FDA pediatric timeline
Policy makers face a tough balance between broad access and targeted protection. Delays in vaccine updates raise questions about equity and whether the most vulnerable will get timely protection, especially older adults and pregnant people with chronic illnesses. Narrowing access to vaccines could widen gaps just as many Americans return to indoor gatherings and schools. A clear, predictable timeline is essential to preserve public trust and keep distribution efficient.
Public health officials must communicate a precise plan that avoids patient confusion and unnecessary cost barriers. If messaging feels slow or opaque, uptake could falter even as the scientific case for vaccines grows stronger. Insurers and healthcare providers play a crucial role in delivering shots, so cost coverage rules and logistics will influence how many people actually get updated vaccines. In the longer run, a steady cadence of updates and transparent priorities will matter as much as the vaccines themselves.
Highlights
- Vaccines timing matters more than crowd size
- Delays in updates create a gap the virus can exploit
- Protecting the at risk remains the backbone of any plan
- Clear timing and access are the core tests of policy
Public health policy and access risk
Delays in vaccine updates and a narrowed initial rollout raise concerns about equity, budget impact, and public reaction if certain groups face barriers to protection.
Clear, predictable vaccination plans will matter as much as the vaccines themselves.
Enjoyed this? Let your friends know!
Related News
Covidwave update prompts health guidance
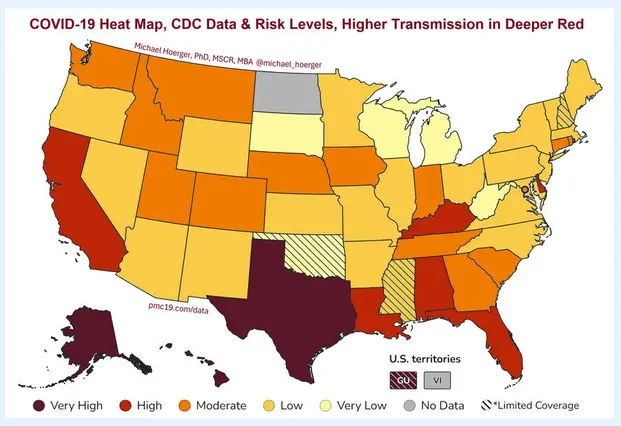
Colorado expands vaccine guidance options
AAP updates vaccine guidance for young children

AAP updates vaccine guidance for toddlers

US cuts funding for mRNA vaccine research

Covid cases are rising across the U.S.
COVID surges in the Southwest
Covid-19 vaccine policy changes spark public concern