T4K3.news
AAP updates vaccine guidance for young children
The American Academy of Pediatrics releases updated guidance on Covid-19 vaccines for infants and children, signaling a shift from CDC recommendations.
The American Academy of Pediatrics updates vaccine guidance to include Covid-19 shots for infants and children, diverging from CDC recommendations.
AAP backs Covid-19 vaccines for young children despite CDC stance
On Tuesday the American Academy of Pediatrics released updated immunization guidance, recommending Covid-19 vaccines for children in specific age groups and risk categories. The AAP says all children 6 months through 23 months should be vaccinated unless they have allergies to the vaccine or its ingredients, and it recommends a single dose for children 2 through 18 years who are at high risk, live in long-term care facilities, have never been vaccinated against Covid-19, or live with high-risk household members. The guidance is more prescriptive than the CDC’s current approach, which relies on shared clinical decision-making rather than a blanket requirement. The FDA has signaled that future Covid-19 shots could be limited to older or higher-risk groups and may not renew authorization for Pfizer’s vaccine for children under 5.
Key Takeaways
"Pediatricians know how important routine immunizations are in keeping children, families and their communities healthy and thriving."
Quote from AAP President Dr. Susan J. Kressly in the news release.
"The AAP will continue to provide recommendations for immunizations that are rooted in science."
Statement in the AAP news release.
"Access to vaccines may be difficult in the fall."
FDA notes potential limits on fall vaccine rollout.
The split between the AAP and CDC highlights how medical groups manage uncertainty and political pressure while trying to protect public health. A more prescriptive stance can simplify counseling for clinicians but risks sparking confusion if families see shifting messages from national agencies. As fall approaches, the practical challenge will be turning guidance into ready access for families who must navigate eligibility and scheduling.
Highlights
- Guidance must evolve with science.
- Trust comes from clear steady messaging.
- Families need practical immunization paths.
- Public health needs coordination across agencies.
Vaccine guidance sparks policy tensions
The divergence between the AAP and CDC on Covid-19 vaccination for children highlights potential public confusion and political sensitivities ahead of fall immunization campaigns.
Public health guidance must turn into practical protection for children, families, and communities.
Enjoyed this? Let your friends know!
Related News

AAP updates vaccine guidance for toddlers
AAP backs COVID vaccine for infants amid clash with Kennedy and CDC
AAP diverges from CDC on child COVID vaccines
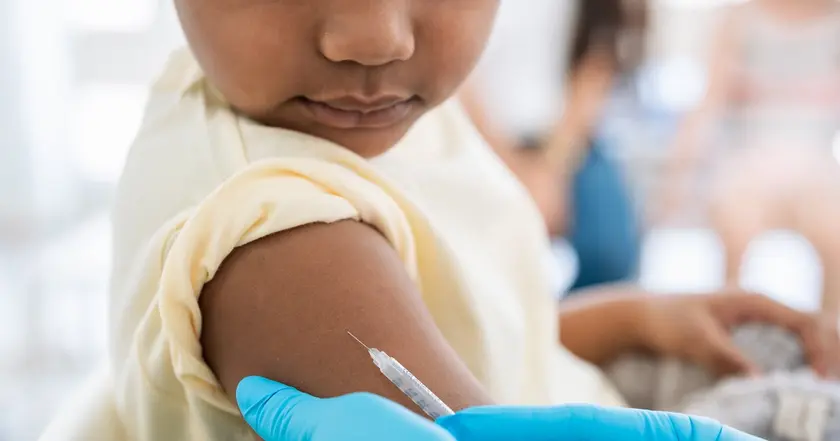
AAP Diverges from CDC on Covid Shots for Children
Covid vaccine update timeline
FDA reviews Pfizer vaccine for under five

AAP challenges Kennedy on COVID vaccines
AAP breaks with federal Covid guidance for babies