T4K3.news
TAR-200 shows strong phase 2 results in bladder cancer
A phase 2 trial finds TAR-200 eliminates tumors in 82% of high‑risk non‑muscle‑invasive bladder cancer patients who had failed prior treatment in three weeks per cycle.

Los Angeles report: a phase 2 trial shows TAR-200 eliminates tumors in 82 percent of high‑risk non‑muscle‑invasive bladder cancer patients who had failed prior treatment.
New treatment eliminates bladder cancer in 82% of patients
A phase 2 study tracked TAR-200, a tiny, pretzel‑shaped device that releases the chemotherapy drug gemcitabine in the bladder over three weeks per cycle. In 85 patients across 144 sites, 70 saw their cancer disappear after treatment, and nearly half remained cancer‑free a year later. Patients already treated with the standard immunotherapy Bacillus Calmette-Guérin (BCG) but whose cancer had returned were eligible, making the group a difficult test case. Doctors described the approach as less invasive than bladder removal and potentially more tolerable given the usually limited efficacy of current options.
The trial followed a schedule of six months of TAR-200 treatment every three weeks, then four maintenance doses per year for the next two years. The regimen was generally well tolerated, with few serious side effects. When TAR-200 was paired with the immunotherapy cetrelimab, results were not as strong and side effects rose, suggesting the pill‑like device works best on its own. With the FDA giving TAR-200 priority review, researchers and patients face heightened hope and a clear question: will the therapy prove durable and scalable in broader use? The device is manufactured by Johnson & Johnson, adding industry interest to the potential rollout.
Key Takeaways
"Our mission is to deliver cancer-fighting medications into the bladder that will offer lasting remission from cancer, and it looks like we are well on our way toward that goal."
Daneshmand on the broader aim of the slow-release approach
"We are at an exciting moment in history."
Daneshmand after reporting early trial success
"The longer the medicine sits inside the bladder, the more deeply it would penetrate the bladder."
Mechanism behind TAR-200’s design
This trial highlights a growing interest in localized, slow‑release therapies that aim to spare patients from more radical surgery. A clear strength is its multi‑center design, reflecting diverse real‑world settings and patient backgrounds. Yet the results come from a phase 2 study with a relatively small cohort. A durable, long‑term remission beyond two years and confirmation from larger, more diverse populations remain essential before widespread adoption. If TAR-200 proves effective in larger trials, it could shift budgets and care pathways, pushing institutions to invest in catheter‑based delivery systems and follow‑up infrastructure. The broader takeaway is a shift toward treatments that focus on disease control within the organ rather than immediate transplantation or invasive procedures. The field should stay cautious and watch for cost, accessibility, and insurance coverage as trials move forward.
Highlights
- Slow release could offer lasting remission
- Longer exposure inside the bladder means deeper effect
- We are at an exciting moment in history
- This changes how we think about treating local cancer
Better science is bringing kinder options for patients who face tough choices about bladder cancer treatment.
Enjoyed this? Let your friends know!
Related News
IO Biotech melanoma trial signals strong PFS but misses primary significance
Pancreatic cancer vaccine shows early promise
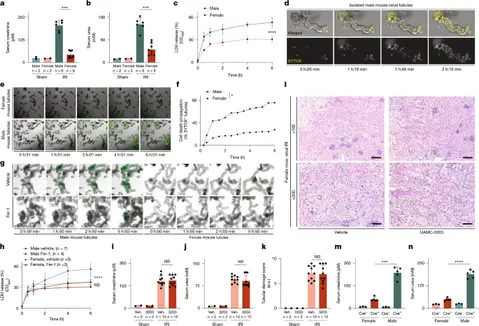
Estrogen protects kidneys from ferroptosis

Stock Markets Climb as Earnings Reports Approach
Off the shelf cancer vaccine shows early promise
Prostate cancer screening gains public support

Mortgage approvals increase as housing market stabilizes

Remedy reports mixed Firebreak performance
