T4K3.news
Ozempic vulva reports prompt safety review
Unverified reports prompt review of GLP-1 medicines and urges patients to report side effects through official channels.

Editorial analysis of reports about a potential side effect linked to Ozempic and similar medicines, focusing on safety signals and medical guidance.
Ozempic vulva prompts safety review of weight loss drugs
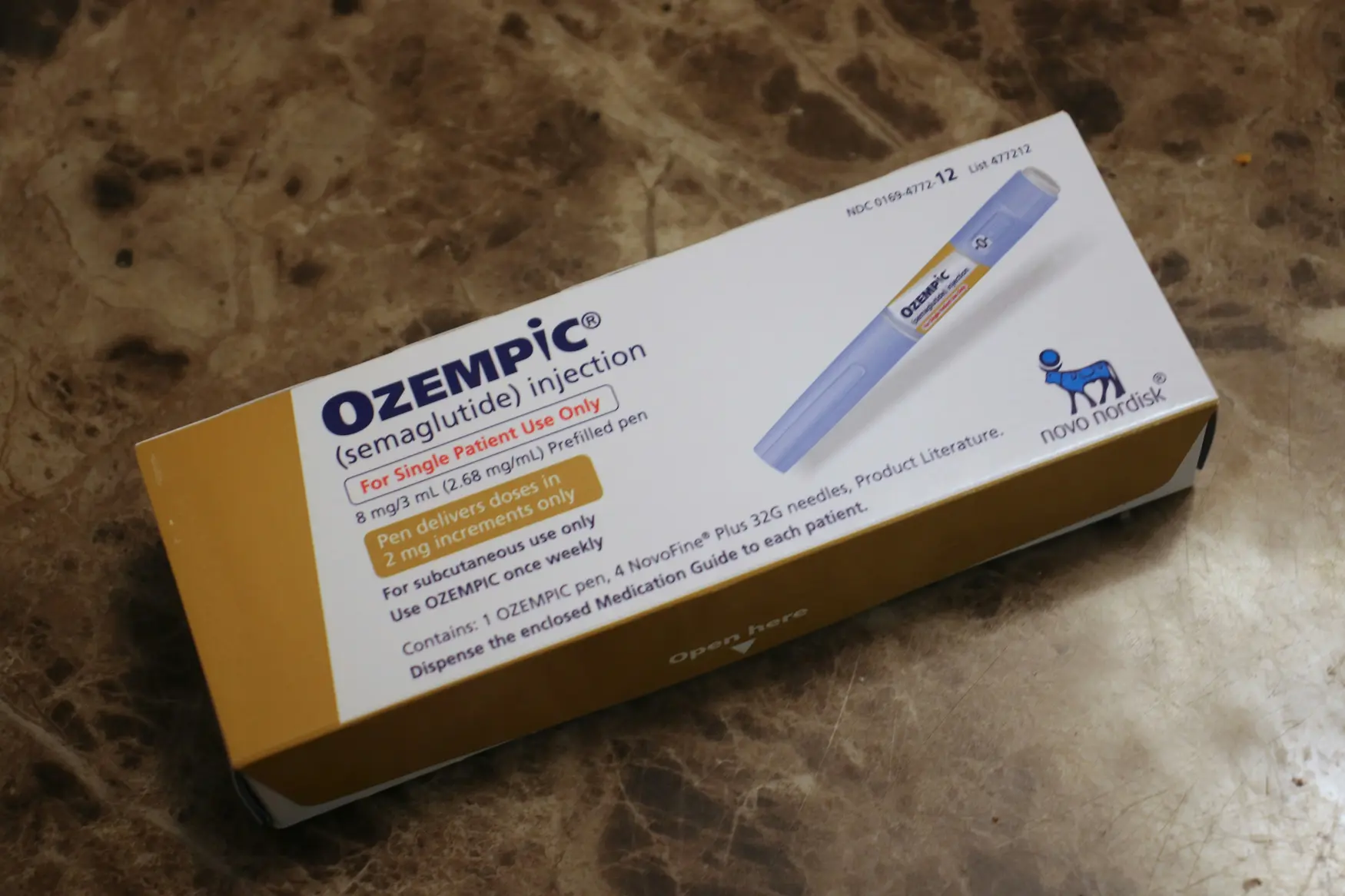
News reports describe a term called Ozempic vulva, claimed by several women as a side effect of GLP-1 medicines that include Ozempic, Wegovy and Mounjaro. The term is not officially recognised by manufacturers. Experts say reports are based on anecdotes rather than official safety data. The drugs are used for diabetes and weight management and work by slowing digestion and reducing appetite. Side effects such as nausea and stomach pain are more widely reported.
Some doctors say rapid fat loss could affect the genital area, causing sagging or deflation in rare cases. Regulators like the MHRA in the UK urge patients to report side effects via the Yellow Card scheme, and Novo Nordisk stresses use only for approved indications under medical supervision. The story shows how online conversations can outpace formal safety signals and the importance of cautious, evidence based guidance.
Key Takeaways
"Loss of subcutaneous fat is global, including the mons pubis and labia majora."
Dr Justin Perron on a possible mechanism
"Patient safety is of the utmost importance to Novo Nordisk."
Company safety commitment
"We recommend patients take these medications only for approved indications and under a healthcare professional."
Official guidance from the company
"Changes to hormone levels and dehydration can lead to vaginal dryness and discomfort."
Gynaecologist’s note on side effects
The piece shows how quickly patient experiences can become headlines, even when evidence is limited. It highlights a tension between patient voices and the evidence base for rare side effects, and it invites readers to consider how media framing may influence people who rely on GLP-1 medicines for weight management. There is a real risk of stigma or misinformation taking hold when unverified reports spread online.
Policy and practice will matter as GLP-1 drugs become more common. Regulators need clear, balanced information about potential side effects, and clinicians must help patients set realistic expectations while monitoring for changes. The balance between broad access to helpful medicines and rigorous safety oversight remains a ongoing challenge for health systems.
Highlights
- Ozempic vulva is not officially recognised by manufacturers
- We recommend patients take these medications only for approved indications and under a healthcare professional
- Loss of subcutaneous fat is global including the mons pubis and labia majora
- Changes to hormone levels and dehydration can lead to vaginal dryness and discomfort
Safety concerns around unofficial side effects of GLP-1 medicines
The article cites unverified reports of a genital side effect linked to Ozempic and related drugs. Regulators emphasize formal reporting and manufacturers stress approved indications. Without robust data, there is a risk of misinformation and patient anxiety.
Time and data will determine how safety guidance evolves.
Enjoyed this? Let your friends know!
Related News
Lawsuits Rise Over Ozempic and Zepbound Safety

Ozempic linked genital changes fuel cosmetic demand

Fort Stewart Shooting Suspect Identified

Arizona child abuse tragedy prompts protection system review

Rabies risk at Grand Teton Lodge

Two drownings at Bahamas resort prompt safety review

Times Square shooting prompts safety questions

Man dies after medical emergency near Coventry Primark
