T4K3.news
Eye risk linked to weight loss jabs prompts checks
New studies link GLP-1 drugs Wegovy and Mounjaro to a modest rise in diabetic retinopathy risk; doctors urge regular eye exams.
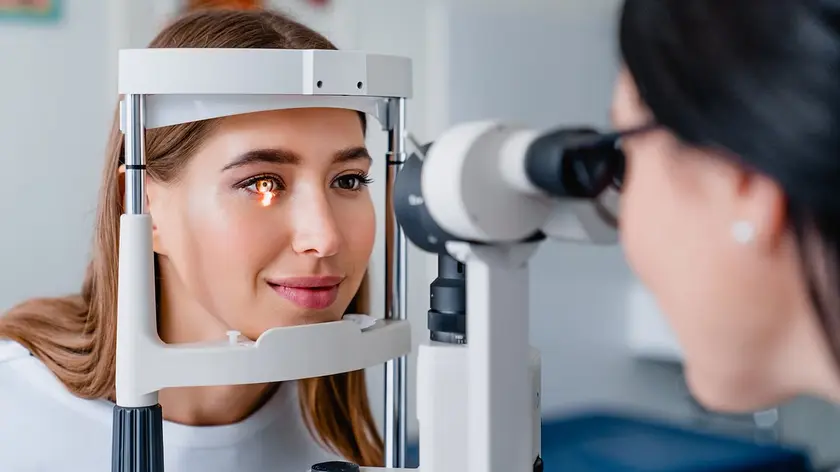
Two studies link GLP-1 drugs Wegovy and Mounjaro to a modest rise in diabetic retinopathy, prompting calls for routine eye checks.
Weight loss jabs raise risk of eye disease
A Massachusetts study of health records from more than 185 000 patients prescribed GLP-1 receptor agonists found a modest link to diabetic retinopathy, a sight threatening complication caused by damage to retinal blood vessels. Drugs in this class include semaglutide, marketed as Wegovy, and tirzepatide, sold as Mounjaro. Researchers noted that while the risk appeared higher in users, the progression to the most severe outcomes was less common than expected, and NAION risk did not rise significantly.
A separate US study with about 159 398 people reported an increased risk of non-arteritic anterior ischaemic optic neuropathy and other optic nerve disorders among those treated with semaglutide or tirzepatide, though the overall incidence remained small. After two years, 0.04% of treated participants developed NAION compared with 0.02% in a matched control group, and 0.12% faced other optic nerve problems versus 0.07% in controls. The researchers cautioned that no association was found with other disorders of the optic nerve or visual pathways. In the Massachusetts study, some authors disclosed conflicts of interest with drug manufacturers.
Key Takeaways
"There is a modestly increased risk of diabetic retinopathy"
Massachusetts study finding
"Overall risk was low"
Second study finding
"Regular eye checks are advised for patients on GLP-1 drugs"
Authors' recommendation
"The balance of benefit and risk needs careful discussion"
Editorial takeaway
Taken together, the findings temper the hype around these weight loss injections. The appeal of rapid pounds off and better blood sugar is clear, but safety signals require clearer guidance for clinicians and patients. The studies highlight the need for independent research and transparent disclosures, because conflicts of interest can shake trust in medical findings.
Regulators and clinicians must balance encouraging obesity treatment with guarding eye health. Routine eye screenings for users, clearer labeling, and patient education could help. The commercial push, high demand, and reports of side effects add pressure on health systems to ensure access without sacrificing safety.
Highlights
- Eyes must come first as pounds come off
- Safety should guide every weight loss claim
- The balance of benefit and risk needs careful discussion
- Independent research is essential for trust
Eye safety concerns tied to weight loss injections
Two independent studies link GLP-1 drugs to a modest rise in diabetic retinopathy risk, with one study noting disclosed conflicts of interest. A second study reports increased risk of optic nerve disorders. Regulatory reviews and high public interest underscore the need for transparent safety data and routine eye screening for users.
Safer use comes from better evidence, not bold promises.
Enjoyed this? Let your friends know!
Related News

Hair loss linked to weight loss jab raises concerns
Ozempic-like medications connected to risk of sudden blindness

Weight loss drug raises mental health alarms

Families overlook key health precautions for summer travel

Study links Pfizer COVID vaccine to eye damage

Investigation launched after deaths linked to weight-loss jabs in Scotland

Liver cancer cases could double by 2050

Rising MASLD affects millions in the UK
